"Skinny Label" patent dispute
- IP News Bulletin
- Jul 21, 2022
- 2 min read
Teva Pharmaceuticals USA Inc. has petitioned to the United States Supreme Court asking the court to overturn the decision in the patent dispute case with GlaxoSmithKline LLC.
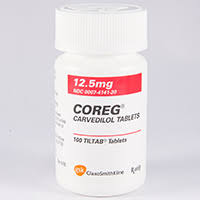
The suit arose over a drug produced by Teva that was a ‘generic’ version of GSK’s heart medication Coreg which treated high blood pressure, the patent for the substance had expired in 2007. Teva released its generic drug on the same year, with a skinny label as certain uses of the drug were patent protected till 2015.
A skinny label is a patent exception, which allows producers of generic medicine to produce and market medicines and seek approval for the same for unpatented uses of the drug. This allows generic drug manufacturers to avoid patent infringement and avoid delays in the release of generic drugs in the market. This avenue has allowed drug makers to get their generic drugs approved.
Branded drug manufacturers have however argued that pharmacists often ignore skinny labels, which could amount to infringement.
GSK first filed a patent infringement suit in 2017 in a Delaware Court, the issue was also raised in the US Patent and Trademarks Office in 2008. The Delaware court found that the skinny label on Teva’s generic drug encouraged doctors to use the drug for heart failure, thus amounting to infringement.
The decision was overturned by a District Court Judge but was again reinstated by a US Appeals Court in 2020. The US Court of Appeals denied Teva’s request to reassess the dispute in February 2022.
Teva argues that this decision is dangerous for generic drug manufacturers as it could lead to many lawsuits being filed against generic drugs bearing skinny labels which have been available on the market for a while without any dispute. Denying Teva’s petition could also weaken the skinny label exception that had been facilitated by the US Congress, which was aimed towards reducing drug spending and increasing availability and affordability of medicines.










Comments